SOCIAL MEDIA
Portuguese Medical Association's Scientific Journal
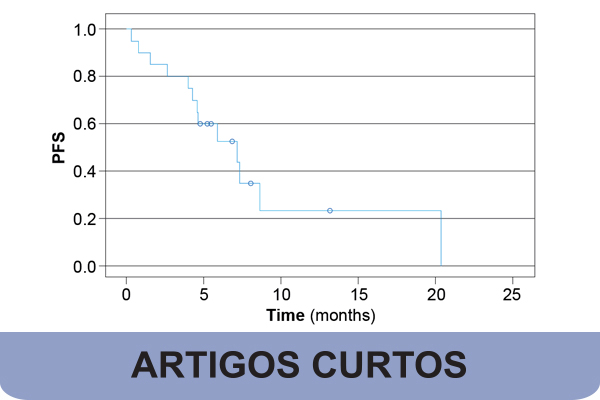
Small cell lung cancer (SCLC) is an aggressive type of lung cancer. Recent studies have provided a new hope by adding atezolizumab to the standard treatment of extensive disease SCLC (E-SCLC). The aim of our study was to evaluate the real-life performance of atezolizumab plus chemotherapy in extensive stage SCLC in a Portuguese setting. Data was collected on twenty patients (70% were male with a mean age of 66.9 years) in treatment at a tertiary hospital in Portugal with E-SCLC treated with chemotherapy and atezolizumab between July 2022 and February 2024. All patients received a carboplatin plus etoposide regimen in combination with atezolizumab. The overall response rate was 55% (95% CI: 31.5 – 76.9) and the disease control rate was 70% (95% CI: 45.7 – 88.1). The median overall survival (OS) and progression-free survival (PFS) was 9.7 (95% CI:5.08 – 14.32) and 7.17 (95% CI:3.28 – 11.05) months, respectively. In total, 13 (65%) patients experienced disease progression and 10 (50%) died during follow-up from events related to the disease. Patients with a performance status score ≥ 2 had lower PFS (p = 0.003) and OS (p = 0.001). To the best of our knowledge, this is the first real-world clinical study in Portugal to evaluate real life outcomes for this combination therapy.