SOCIAL MEDIA
Portuguese Medical Association's Scientific Journal
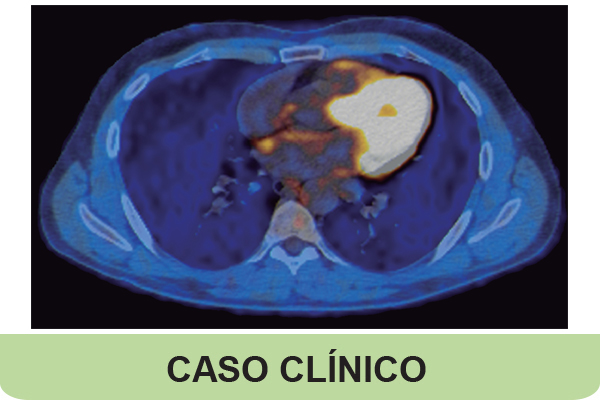
Lung cancer has a high mortality rate; however, treatment with tyrosine kinase inhibitors targeting specific molecular alterations has significantly improved the survival of patients with advanced or metastatic non-small cell lung carcinoma (NSCLC). EGFR mutations are present in approximately 15% of NSCLC cases. Osimertinib was approved in Portugal by Infarmed (Portuguese Medicines Agency) in 2021 as a first-line therapy for advanced NSCLC with EGFR sensitizing mutations. A 55-year-old man, a former smoker, presented to the Emergency Department with a six-month history of dry cough and dyspnea that had worsened and was now accompanied by fever. Chest CT revealed multifocal pulmonary consolidations that were already present in a scan performed three months earlier. Bronchial biopsies confirmed a diagnosis of lung adenocarcinoma with an EGFR exon 19 deletion. Staging tests revealed stage IV-A disease (pulmonary metastasis and, later, right adrenal metastasis identified on PET-FDG). The patient was started on osimertinib. He was discharged and progressively recovered his baseline general condition, achieving a performance status of 0 and resuming physical activity. Despite the extensive thoracic disease, the patient achieved a complete metabolic response documented on PET-CT five months after initiating therapy, along with significant clinical improvement. Osimertinib effectively inhibits the EGFR signaling pathway and has been established as the firstline treatment for patients with stage IV disease since the FLAURA trial. However, such complete responses are rare and raise further questions about the factors influencing these responses, the optimal duration of therapy in these cases, and the role of circulating tumor DNA in therapy monitoring and discontinuation decisions.